Apollo Gleneagles Hospitals
58 Canal Circular Road,
Kolkata - 700054, WB, India.
Contact : +91 9874167730 / +91 9830363642
Email : pchakrabarti@usa.net

Apollo Gleneagles Hospitals
58 Canal Circular Road,
Kolkata - 700054, WB, India.
Contact : +91 9874167730 / +91 9830363642
Email : pchakrabarti@usa.net
The biggest problem facing transplant centers today is the shortage of organ donors. Mechanisms that might increase the number of available organs include:
(a) Optimizing the current donor pool (e.g., the use of multiple organ donors or marginal donors);
(b) increasing the number of living-donor transplants (e.g., the use of living unrelated donors);
(c) using unconventional and controversial donor sources (e.g., using deceased donors without cardiac activity or anencephalic donors);
(d) performing xenotransplants. The largest potential increase in the number of available organs would result from improving donation rates from suitable deceased donors. By recent estimates, over 10,000 brain-dead donors are potentially available in the United States annually. Currently, however, only about one half of them are actually used. The single most important reason for the lack of deceased-donor organ retrieval is the inability to obtain consent from the surviving next-of-kin. The need for public education is crucial, including more effective educational campaigns to increase awareness of the importance of organ transplants.
Most extrarenal transplants performed today, and roughly one half of all renal transplants, are from deceased donors. These donors are deceased individuals who meet the criteria for brain death, but whose organs are being perfused by life-support measures, allowing adequate time for referral to an organ procurement organization. A member of that organization can then ascertain whether donation is possible, and if so, approach the potential donor's family and possibly obtain consent to procure suitable organs. Crucial to the concept of deceased-donor organ donation is the concept of brain death. Brain death means that all brain and brain stem function has irreversibly ceased, while circulatory and ventilatory functions are maintained temporarily. The clinical diagnosis of brain death rests on three criteria: (a) irreversibility of the neurologic insult; (b) absence of clinical evidence of cerebral function; and most important, (c) absence of clinical evidence of brain stem function. When testing for brain death, hypothermia, medication side effects, drug overdose, and intoxication must be excluded. Brain death can be diagnosed by routine neurologic examinations (including cold caloric and apnea testing on two separate occasions at least 6 hours apart), coupled with prior establishment of the underlying diagnosis. Confirmatory tests must verify the absence of intracranial blood flow on brain flow studies or the presence of an isoelectric electroencephalogram reading. Once the diagnosis of brain death has been established, the process of organ donation can be initiated. 30,31 The focus then switches from the treatment of elevated intracranial pressure (ICP) to preserving organ function and optimizing peripheral oxygen delivery.32 It is important to keep in mind that management of the deceased organ donor is an active process, requiring aggressive monitoring and intervention to ensure that perfusion to the organs of interest is not compromised. For all organ donors, core temperature, systemic arterial blood pressure, arterial oxygen saturation, and urine output must be determined routinely and frequently. Arterial blood gases, serum electrolytes, blood urea nitrogen, serum creatinine, liver enzymes, hemoglobin, and coagulation tests also need to be monitored regularly. Hemodynamic instability can be marked after brain death, with wide swings between the extremes of hypotension and hypertension. Hypotension is usually secondary to hypovolemia, due to a combination of vasomotor collapse after brain death and the effects of treatment protocols to decrease ICP. Hypertension may also be seen, often secondary to raised ICP. It can be treated with short-acting vasodilatory agents or with rapidly reversible beta blockers. Other key factors in donor management include respiratory maintenance, good renal perfusion with brisk urine output, and avoidance of hypothermia.
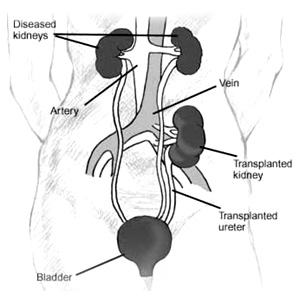
The technique of multiple organ procurement (kidney, liver, pancreas, small bowel) was first described by Starzl and his colleagues in 1984. 33 Most centers have now added their own modifications to these pioneering techniques and differ primarily on their degree of in vivo dissection. Some centers perform extensive dissection of the organs to be recovered before flushing the organs with preservative solution. Other centers prefer to flush the organs early, remove the abdominal contents "en-bloc," and perform the separation and dissection of the individual organs on the back table.34 Each technique has its potential advantages and disadvantages. Regardless of personal technique and preference, it is paramount that the transplant surgeon develops a systematic approach to safely procure the liver, pancreas, and kidneys even in the unstable donor.
The basic steps involve a long incision to provide wide exposure of all thoracic and abdominal organs. Complete mobilization of the distal small bowel, right colon, and duodenum is performed to allow for identification of the distal aorta, iliac bifurcation, and the distal inferior vena cava (IVC). The infrarenal aorta will serve as the site for insertion of the cannula that will allow for flushing of the organs with cold preservative solution . The supraceliac aorta is encircled followed by limited dissection of the hepatic hilum and the pancreas. The portal system can be cannulated via the inferior mesenteric vein and the organs can then be flushed with preservative solution and topically cooled with slush. The thoracic organs, liver, pancreas, and kidneys are then removed individually.
The non-heart-beating donor (NHBD), also referred to as the donation after cardiac death donor, is one type of expanded criteria donor that is increasingly being used by transplant centers to successfully boost the number of deceased donors and decrease the dire shortage of transplantable organs.35 NHBD death is characterized by irreversible absence of circulation, in contrast to heart-beating donor death, defined by irreversible cessation of all brain functions. Organ ischemia is minimized in the brain-dead donor because circulatory arrest typically occurs concurrently with perfusion of preservation solution and rapid core cooling. NHBDs are less than ideal because the organs suffer ischemia during the prolonged periods between circulatory dysfunction, circulatory arrest, and subsequent perfusion and cooling. Furthermore, the surgical procedure for NHBD organ recovery is demanding and rushed.
It is important to differentiate controlled from uncontrolled NHBDs. Uncontrolled NHBDs sustain circulatory arrest and either fail to respond to cardiopulmonary resuscitation and/or are declared dead on arrival to the hospital. Uncontrolled NHBD death is unplanned, so the organs suffer protracted ischemia before recovery. Although kidneys tolerate a short period of the resultant warm ischemia, transplantation of extrarenal organs from uncontrolled NHBDs carries a much greater risk. In contrast, controlled NHBDs undergo circulatory arrest following planned withdrawal of life support, most often in the operating room, with a donor surgical team readily available. Controlled NHBDs suffer terminal illness, usually a severe neurologic injury without the possibility of meaningful recovery or survival. Controlled NHBDs provide organs that are exposed to significantly less ischemic damage than those of uncontrolled NHBDs and, in general, offer superior posttransplant function when compared with uncontrolled NHBDs.
Living-donor transplantation is unique in that surgeons are operating on a healthy individual (i.e., a living donor) who has no medical disorders and does not require an operation. The use of living donors is an integral and important part of the field of transplantation today. The first transplants ever performed used living donors. Today, living donors are commonly used for every type of transplant except heart transplants. The number of such transplants continues to increase on a yearly basis. But living-donor transplants pose a unique set of medical, ethical, financial, and psychosocial problems that must be dealt with by the transplant team. The use of living donors offers numerous advantages. Primary is the availability of a life-saving organ. A certain percentage of transplant candidates die while waiting for a deceased-donor organ as a direct result of a complication, or of progression of their underlying disease. For such ill candidates, the advantage of a living donor is obvious. In certain parts of the world, such as the Far East, where deceased-donor transplants are not accepted by the public, the advantage and need of the living donor is obvious. Even in countries where deceased-donor transplants are accepted, a living-donor transplant may significantly shorten the waiting time for potential recipients. A shorter waiting time generally implies a healthier candidate-one whose body has not been ravaged by prolonged end-stage organ failure. Moreover, living-donor transplants are planned (rather than emergency) procedures, allowing for better preoperative preparation of the potential recipient. Receiving an organ from a closely matched relative may also have immunologic benefits. Lastly, long-term results may be superior with living-donor transplants, which is certainly the case with kidney transplants. The disadvantages of a living-donor transplant for the potential recipient are minimal. With some organ transplants (e.g., living-donor liver or lung), the procedure may be more technically complex, resulting in an increased incidence of surgical complications. However, this disadvantage is offset by the numerous advantages. The major disadvantage of living-donor transplants is to the donor. Medically, there is no possibility of benefit for the donor, only potential for harm. The risk of death associated with donation depends on the organ being removed. For nephrectomy, the mortality risk is estimated to be less than 0.05%. However, for partial hepatectomy, it is about 0.5%. Risks for surgical and medical complications also depend on the procedure being performed. In addition, long-term complications or problems may be associated with partial loss of organ function through donation. The guiding principle of all living-donor transplants should be the minimization of risk to the donor. What risk there is must be carefully explained to the potential donor, and written informed consent should be obtained. The kidney, the first organ to be used for living-donor transplants, is the most common type of organ donated by living donors today. Living-donor liver transplants are not as common, but have been performed for almost 15 years now. Initially, they involved adult donors and pediatric recipients, but now an adult donor for an adult recipient is more common. Living-donor transplants with organs besides the kidney and liver are fairly uncommon, but are performed at various centers. Living-donor pancreas transplants involve a distal pancreatectomy, with the graft consisting of the body and tail of the pancreas; vascular inflow and outflow are provided by the splenic artery and splenic vein. Living-donor intestinal transplants usually involve removal of about 200 cm of the donor's ileum, with inflow and outflow provided by the ileocolic vessels. Living-donor lung transplants involve removal of one lobe of one lung from each of two donors; both grafts are then transplanted into the recipient.
Organ preservation methods have played an important role in the success of cadaver-donor transplants. They have resulted in improved graft function immediately posttransplant and have diminished the incidence of primary nonfunction of organs. By prolonging the allowable cold ischemia times, they have also allowed for better organ allocation and for safer transplants.36,37 The most common methods involve the use of hypothermia and pharmacologic inhibition to slow down metabolic processes in the organ once it has been removed from the deceased donor. Hypothermia very effectively slows down enzymatic reactions and metabolic activity, allowing the cell to make its limited energy reserves last much longer. A temperature decrease from 37° to 4°C (98.6° to 39.2°F) (the temperature of most preservation solutions) slows metabolism about 12-fold. However, in the absence of any energy inflow into the cell, degradative reactions begin to provide the cell with an energy source. The result can be destruction of important structural elements and, eventually, structural damage to the cells and the organ. So, although hypothermia greatly slows enzymatic reactions, they continue nonetheless, leading to accumulation of potentially detrimental end products within the cell. Hypothermia also contributes to the development of cellular swelling because the membrane ion pumps are slow to function. Cold storage solutions have been developed to improve organ preservation by ameliorating some of the detrimental effects of hypothermia alone. Essentially, these solutions suppress hypothermia-induced cellular swelling and minimize the loss of potassium from the cell. Agents that do not readily permeate the cell membrane and that have an electrolyte composition resembling the intracellular environment (low sodium, high potassium) are used, thus preventing the loss of cellular potassium. The most commonly used fluid worldwide is the University of Wisconsin solution.38 It contains lactobionate, raffinose, and hydroxyethyl starch. Lactobionate is impermeable and prevents intracellular swelling; it also lowers the concentration of intracellular calcineurin and free iron, which may be beneficial in reducing reperfusion injury. Hydroxyethyl starch, a synthetic colloid, may help decrease hypothermia-induced cell swelling of endothelial cells and reduce interstitial edema. Another solution that is now being used commonly is histidine-tryptophan-ketoglutarate solution.39 Although cold preservation has improved cadaver-donor transplant results, the amount of time that an organ can be safely preserved is limited. After that, the incidence of organ nonfunction starts to increase. With kidneys, exceeding the preservation time limit results in delayed graft function, requiring dialysis support for the recipient until function improves. With livers, the result is primary nonfunction, requiring an urgent retransplant. How long an organ can be safely preserved depends on the type of organ and on the condition of the donor. With kidneys, cold ischemic times should be kept below 36 to 40 hours; after that, delayed graft function significantly increases. With pancreata, more than 24 hours of ischemia increases problems due to pancreatitis and duodenal leaks. With livers, more than 16 hours of ischemia increases the risk for primary nonfunction and biliary complications. Hearts and lungs tolerate preservation poorly; ideally, ischemia times should be below 6 hours. With marginal donors, all of these times should be adjusted further downward.